Introduction: Idelalisib, a selective oral inhibitor of PI3Kδ, was approved by the FDA and EMA as monotherapy for patients with advanced follicular lymphoma (FL) based on positive efficacy and safety results from the registration phase 2 trial in patients with indolent non-Hodgkin lymphoma (iNHL) refractory to 2 prior regimens (NCT01282424; 101-09). Phase 3 studies of idelalisib in earlier lines of treatment for iNHL were terminated prematurely due to an increased risk of death and higher incidence of serious adverse events (AEs) associated with idelalisib. Herein, we present the preplanned interim results as of June 8, 2020, of the safety of idelalisib monotherapy in patients with refractory FL treated in the EU from one of the largest noninterventional trials of idelalisib.
Methods: This was a noninterventional, partially retrospective cohort study conducted in 10 European countries (EUPAS19618; NCT03568929). Eligible patients were adults aged ≥18 years treated with idelalisib for refractory FL, per the Summary of Product Characteristics and treatment guidelines, in routine clinical practice. Data was collected from patients' medical records and anonymized. Safety events were evaluated and included treatment-emergent AEs (TEAEs), health outcomes of interest (HOIs) requested by the EMA (bowel perforations, Grade ≥3 diarrhea/colitis, progressive multifocal leukoencephalopathy, pneumonitis, Grade ≥3 rash, infections, Stevens-Johnson syndrome, transaminase elevations, and hepatocellular injury), and deaths. TEAEs were defined as AEs that occurred between first dose and 30 days after last dose. Data were summarized using descriptive statistics. Rates per person-year were calculated as the number of patients experiencing an AE divided by the person-time at risk.
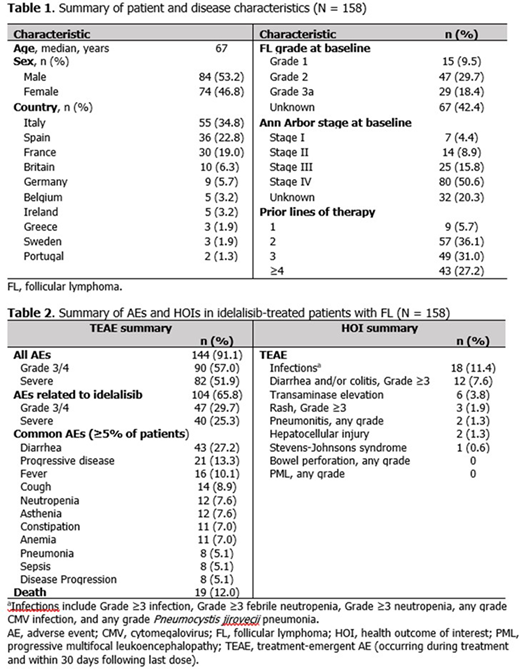
Results: For the 158 patients included in this analysis, median age was 67 years and 84 (53%) were male. A summary of patient and disease characteristics at baseline are presented in Table 1. At treatment initiation, 47 (30%) patients had grade 2 FL and 105 (66%) had Ann Arbor stage III/IV disease, though these characteristics were not captured for 42% and 20% of patients, respectively; 149 (94%) had ≥2 prior therapies. The median duration of idelalisib exposure was 165 (range 11-1321) days, and patients were observed for a median of 323 (range 12-1676) days.
In total, 144 (91%) patients experienced a TEAE, with a rate of 3.36 per person-year. The proportion of patients experiencing Grade 3/4, severe, common, and HOI TEAEs are presented in Table 2. Diarrhea of any grade was the most common TEAE, and infections were the most common treatment-emergent HOI. During the TEAE period, 19 (12%) patients died (Table 2).
The proportion of common TEAEs reported in this analysis were lower compared with a subgroup analysis of patients with FL from the registration trial (Salles Haematologica 2017; DOI: 10.3324/haematol.2016.151738), including diarrhea 27% vs 51%, cough 9% vs 32%, fever/pyrexia 10% vs 29%, pneumonia 5% vs 11%; treatment-emergent Grade 3/4 diarrhea/colitis and transaminase elevations were also lower in this analysis: 8% vs 17% and 4% vs 14%. This comparison is limited due to differences in study populations, namely that patients in the registration trial had more advanced disease (stage III/IV, 66% vs 83%) and had a higher median number of prior therapies (3 vs 4).
Conclusions: This interim analysis of the safety of idelalisib in patients with refractory FL in this large noninterventional safety study provides an estimate of potential final study findings. The AE profile from this study appears to corroborate the known safety profile of idelalisib. No new safety signals were detected.
Salles:Bristol Myers Squibb: Consultancy, Other; Genmab: Consultancy; Gilead: Consultancy, Honoraria, Other: Participation in educational events; Epizyme: Consultancy; Abbvie: Consultancy, Honoraria, Other: Participation in educational events; Autolus: Consultancy; Amgen: Honoraria, Other: Participation in educational events; Celgene: Consultancy, Honoraria, Other: Participation in educational events; Debiopharm: Consultancy; Takeda: Consultancy, Honoraria, Other; Novartis: Consultancy, Honoraria, Other; F. Hoffman-La Roche Ltd: Consultancy, Honoraria, Other; Janssen: Consultancy, Honoraria, Other: Participation in educational events; Karyopharm: Consultancy; Kite: Consultancy, Honoraria, Other; MorphoSys: Consultancy, Honoraria, Other. Pettengell:Celgene: Honoraria; MEI Pharma: Honoraria; Roche Ltd: Honoraria; Takeda: Honoraria. Vandenberghe:Celgene: Other: sponsorship to attend Lugano lymphoma meeting in 2019; Abbvie: Other: travel grants, Research Funding; Gilead: Other: travel grants, Research Funding; Janssen: Other: travel grants; Roche: Other: travel grants, Research Funding. Corradini:Kiowakirin: Consultancy, Honoraria; Incyte: Consultancy; Gilead: Consultancy, Honoraria, Other; F. Hoffman-La Roche Ltd: Consultancy, Honoraria; Daiichi Sankyo: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Other; BMS: Other; Servier: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Other; Kite: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Other; Takeda: Consultancy, Honoraria, Other; AbbVie: Consultancy, Honoraria, Other. De Belder:Gilead Sciences Europe, Ltd.: Current Employment. Shah Gupta:Gilead Sciences, Ltd.: Current Employment. van Troostenburg:Gilead Sciences GmbH: Current Employment. Rajakumaraswamy:Gilead Sciences, Inc.: Current Employment. Ramroth:Gilead Sciences, Ltd.: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal